About SureScreen’s COVID-19 Rapid Test Cassette
This test is CE marked for Professional use only. SureScreen’s COVID-19 rapid test identifies the body’s response to coronavirus after the onset of infection, and gives a qualitative yes/no result within 10 minutes.
Implementing rapid screening for COVID-19 has huge time and cost savings when compared to laboratory screening, and will help to control the spread of the virus by identifying infection rapidly and accurately.
The test cassette is easy to use, needing only a finger-prick sample to function, much like a blood glucose test
As well as whole blood, the cassette can also be run with serum or plasma samples
- Corona – crown-like
- 7 types, 4 common, 3 serious
- SARS-CoV-2 (COVID-19), MERS-CoV (MERS) and SARS-CoV-1 (SARS)
SureScreen’s COVID-19 Rapid Test identifies the body’s response to Coronavirus after the onset of infection, and gives a qualitative yes/no result as to whether antibodies have been produced by the body within 10 minutes.
Over all accuracy 97.8% (IgM) and 99.6% (IgG)
The test cassette is easy to use, can be used at the patient side, and only requires a finger-prick sample to function, much like a blood glucose test. As well as whole blood, the cassette can also be run with serum or plasma samples.
Importantly the test kits are CE marked for Professional use, using all EUROPEAN MANUFACTURED COMPONENTS
Independently validated by:
St. Thomas’s NHS Trust London (treated British Prime Minister Mr Boris Johnson)
Kings College London,
Guy’s NHS Trust London,
UZ Leuven Belgium
The French National Centre for Scientific Research
Cambridge University
Imperial College London
Champalimaud Portugal
Birmingham NHS
Clinical Validation results
The SureScreen Antibody Test Kits thorough independent clinical validations has shown very high levels of Sensitivity.
References:
National Public Health Emergency Team (NPHET) Subgroup (of the Department of Health of Ireland). Diagnostic Testing Approaches A Strategic Framework. Dublin, Ireland: Rialtas na hÉireann (Government of Ireland): 2020 May 22. Available: assets.gov.ie.
Pickering S, Betancor G, Galao RP, Merrick B, Signell AW, Wilson HD, Ik MTK, Seow J, Graham C, Acors S, Kouphou N, Steel KJA, Hemmings O, Patel A, Nebbia G, Douthwaite S, O’Connell L, Luptak J, McCoy LE, Brouwer PJM, van Gils MJ, Sanders RW, Nunez RM, Bisnauthsing K, O’Hara G, MacMahon E, Batra R, Malim MH, Neil SJD, Doores KJ, Edgeworth JD. Comparative assessment of multiple COVID-19 serological technologies supports continued evaluation of point-of-care lateral flow assays in hospital and community healthcare settings. medRxiv. 2020 Jun 4. preprint doi: 10.1101/2020.06.02.20120345.
Health Information and Quality Authority (of Ireland). Rapid health technology assessment of alternative diagnostic testing approaches for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Dublin, Ireland: HIQA; 2020 May 5. Available: www.hiqa.ie/sites/default/files/2020-05/Rapid_HTA_COVID-19_tests.pdf.
Humanity tested. Nat Biomed Eng. Epub 2020 Apr 8. doi: 10.1038/s41551-020-0553-6. PMID: 32269324. PMCID: PMC7139197.
European Commission. Current performance of COVID-19 test methods and devices and proposed performance criteria – Working document of Commission services. Brussels, Belgium: European Commission;2020 Apr 16. Available: ec.europa.eu/docsroom/documents/40805.
Who could Test?
Airport / Borders
Occupational Health workers
Returning back into the workplace post lockdowns / Gov.ie advise
Primary Care Centres / Hospitals /Dentists
Private Healthcare (Nursing Homes)
Where can you get tested?
Your local GP.
Primary Care Centres.
Pharmacies.
Key Benefits
Accurate screening tool (over 98% accuracy)
Rapid 10 minute testing time
Point of care test that can be used by the patient side
Cost effective vs. Laboratory tests
Manufacturer Support direct in the UK
Shown to improve accuracy when used alongside PCR (antigen) testing
Ideal for seroprevalence testing
No need for laboratory equipment
Diagnostic
Diagnostics are essential to understand spread of infection
Long incubation period, and it is thought that 20% of cases are asymptomatic
Testing blood and sputum samples are the main avenues of screening.
Testing can be done to look for the antigen and antibodies
PCR (Polymerase Chain Reaction) main avenue for antigen testing but labs have faced capacity constraints pushing long timelines for results and requires lab technicians.
Serological testing for antibodies using SureScreen’s rapid test allows a quick solution for antibody testing
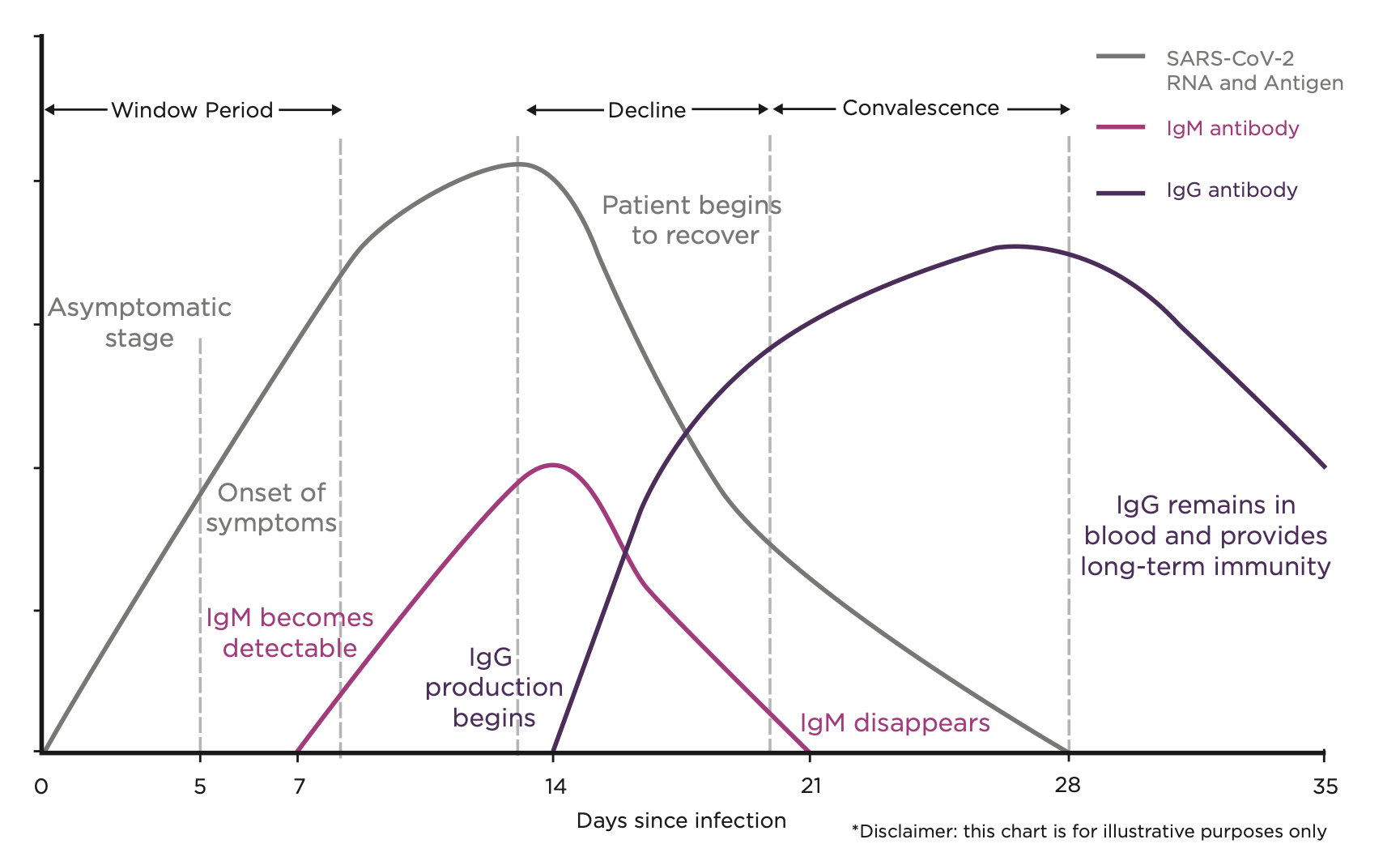
Conclusions
Experts at Guy’s and St. Thomas’s Hospital and Kings College University in London have been carrying out one of the most exhaustive examinations of antibody tests since COVID-19 emerged. They have been comparing the accuracy of tests from different companies and have published data which shows SureScreen’s performed outstandingly in critical areas. The tests have been deemed so good that the prestigious hospital is now using them daily.
The study illustrates how important quality antibody testing is for combatting COVID-19. If used in mass testing, it could be vital in showing how many people had contracted the virus and lead to a better understanding of potential immunity.
The paper states: “There is a clear requirement for accurate serology (blood) testing as a companion diagnostic to PCR-based (swab) testing… Monitoring population seroprevalence (examining who has antibodies) will be central to future public health planning based on disease susceptibility and herd immunity.”
The report goes on to state.: “For this (widescale testing) to be meaningful, it is imperative that antibody detection methods are affordable, reliable, and readily accessible.
Manufactured by: SureScreen Diagnostics, 1Prime Parkway, Prime Enterprise Park, Derby, DE13QB, United Kingdom
